
Background
PFAS Background and Nomenclature Information and Resources
A compilation of Frequently Asked Questions can be found Here (updated 03/24/20)
PFAS General
Per- and polyfluoroalkyl substances (PFAS) are a large group of human-made substances that do not occur naturally in the environment and are resistant to heat, water, and oil. PFAS have been used extensively in surface coating and protectant formulations due to their unique ability to reduce the surface tension of liquids [1]. Perfluorooctane sulfonic acid (PFOS) and perfluorooctanoic acid (PFOA) are two types of PFAS that are no longer manufactured or imported into the United States [2]; however, there could be some imported goods containing trace amounts of these substances[3].
Other PFAS goods and materials are still produced and used in the United States [4]. PFAS are persistent in the environment, can accumulate within the human body over time, and are toxic at relatively low concentrations [5]. Exposure to unsafe levels of PFOA/PFOS may result in adverse health effects including developmental effects to fetuses during pregnancy, cancer, liver effects, immune effects, thyroid effects, and other effects (such as cholesterol changes)[6]. PFOA and PFOS were found in the blood of nearly all people tested in several national surveys[7], [8]. According to the Center for Disease Control (CDC), blood levels of both PFOS and PFOA have steadily decreased in U.S. residents since 1999-2000[9].
PFAS can be introduced into the body by eating or drinking contaminated food or liquid (including water), breathing in or touching products treated with PFAS, such as carpets or clothing [10]. Exposure to PFOA and PFOS is generally dominated by the ingestion of food [11]. Food can be contaminated by the migration of PFAS from packaging [12], and some foods such as fish, meat, eggs and leafy vegetables may contain PFAS due to bioaccumulation and crop uptake [13].Contaminated drinking water has led to high levels of exposure to PFOA, PFOS, and other PFAS for some populations residing
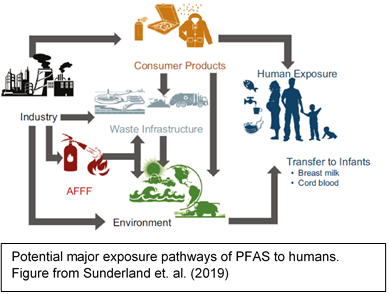
near manufacturing facilities[14]. Workers in facilities that make or use PFAS can be exposed to higher amounts of these chemicals and have higher levels in their blood [15].
Infants may be exposed to PFAS through breastfeeding [16]. However, the benefits of breastfeeding are well known and generally outweigh potential risks from transfer of chemicals [17], but you can talk with your doctor if you have concerns.
PFAS, especially PFOS and PFOA, have been detected in air, water, and soil in and around manufacturing facilities; however, these releases have been declining since companies began phasing out the production and use of several PFAS in the early 2000s[18].Due to their chemical structure, PFAS are very stable in the environment and are resistant to breaking down.
Some PFAS are volatile and can be carried long distances through the air, which may lead to contamination of soils and groundwater far from the source of the PFAS emission [19]. PFAS have been detected in many parts of the world, including oceans and the Arctic, indicating that long-range transport is possible[20].
The four major sources of PFAS are: fire training/fire response sites, industrial sites, landfills, and wastewater treatment plants/biosolids[10]. PFAS can get into drinking water when products containing them are used or spilled onto the ground or into lakes and rivers[21]. Once in groundwater, PFAS are easily transported large distances and can contaminate drinking wells [10]. PFAS in the air can also end up in rivers and lakes used for drinking water[10]. Additional information regarding the fate and transport of PFAS in the environment may be found on the Interstate Technology Regulatory Council.
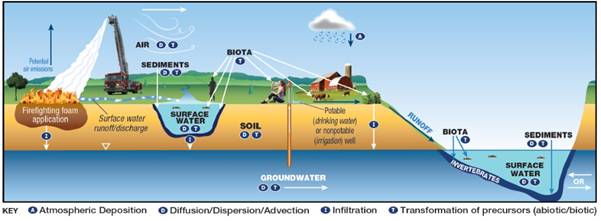
Potential Mechanisms of transport of PFAS from firefighting foam application to environmental media.
PFAS have been used extensively in surface coating and protectant formulations due to their unique ability to repel oil, grease and water. Major applications have included protectants for paper and cardboard packaging products, carpets, leather products, and textiles that enhance water, grease, and soil repellency, and in firefighting foams[22]. PFAS have also been used as processing aids in the manufacture of nonstick coatings on cookware[22].
Under the PFOA Stewardship Program with the U.S. Environmental Protection Agency (US EPA), eight major PFAS producers have phased out PFOA and other PFAS substances from emissions and products[2]. However, manufacturers are developing replacement technologies in the PFAS family by substituting longer-chain substances with shorter-chain substances such as GenX and ADONA [23]. While less information is available for these shorter-chain substances, studies have shown that they behave in a similar toxicological manner as their longer-chain counterparts [24], [25]. Due to the wide range of chemicals within the PFAS family and the observed similarities in their toxic mode-of-action, efforts are underway to calculate relative potency estimates for chemicals within the PFAS class in order to better inform regulation [26].
To complement the PFOA Stewardship Program, US EPA has issued regulations, known as Significant New Use Rules (SNURs), requiring manufacturers and processors of these chemicals to notify US EPA of new uses of these chemicals before they are commercialized[27]. Specifically, the regulations require that anyone who intends to manufacture (including import) or process any chemicals for uses contained in the SNUR must submit a notification to US EPA at least 90 days before beginning the activity[27]. This provides US EPA with an opportunity to review and, if necessary, place limits on manufacturers or processors who intend to reintroduce or import products with these chemicals.
Learn more about EPA’s actions on PFASs and other perfluorinated chemicals.
[1] T. H. Begley, K. White, P. Honigfort, M. L. Twaroski, R. Neches, and R. A. Walker, “Perfluorochemicals: Potential sources of and migration from food packaging,” Food Addit. Contam., vol. 22, no. 10, pp. 1023–1031, Oct. 2005.
[2] US EPA, “PFOA Stewardship Program Docket ID Number EPA-HQ-OPPT-2006- 0621. US Environmental Protection Agency, Washington, DC.,” 2006.
[3] 3M Company, “Fluorochemical use, distribution and release overview.,” AR226-0550, 1999.
[4] H. Lee, J. D’eon, and S. A. Mabury, “Biodegradation of Polyfluoroalkyl Phosphates as a Source of Perfluorinated Acids to the Environment,” Environ. Sci. Technol., vol. 44, no. 9, pp. 3305–3310, May 2010.
[5] Z. Wang, J. C. DeWitt, C. P. Higgins, and I. T. Cousins, “A Never-Ending Story of Per- and Polyfluoroalkyl Substances (PFASs)?,” Environ. Sci. Technol., vol. 51, no. 5, pp. 2508–2518, Mar. 2017.
[6] ATSDR, “Toxicological Profile for Perfluoroalkyls, Draft for Public Comment,” 2018.
[7] A. M. Calafat, L.-Y. Wong, Z. Kuklenyik, J. A. Reidy, and L. L. Needham, “Polyfluoroalkyl Chemicals in the U.S. Population: Data from the National Health and Nutrition Examination Survey (NHANES) 2003–2004 and Comparisons with NHANES 1999–2000,” Environ. Health Perspect., vol. 115, no. 11, pp. 1596–1602, Nov. 2007.
[8] J. M. Graber et al., “Per and polyfluoroalkyl substances (PFAS) blood levels after contamination of a community water supply and comparison with 2013–2014 NHANES,” J. Expo. Sci. Environ. Epidemiol., vol. 29, no. 2, pp. 172–182, Mar. 2019.
[9] CDC, “National Report on Human Exposure to Environmental Chemicals,” 2019.
[10] E. M. Sunderland, X. C. Hu, C. Dassuncao, A. K. Tokranov, C. C. Wagner, and J. G. Allen, “A review of the pathways of human exposure to poly- and perfluoroalkyl substances (PFASs) and present understanding of health effects,” J. Expo. Sci. Environ. Epidemiol., vol. 29, no. 2, pp. 131–147, Mar. 2019.
[11] L. S. Haug, S. Huber, G. Becher, and C. Thomsen, “Characterisation of human exposure pathways to perfluorinated compounds — Comparing exposure estimates with biomarkers of exposure,” Environ. Int., vol. 37, no. 4, pp. 687–693, May 2011.
[12] L. A. Schaider et al., “Fluorinated compounds in US fast food packaging,” Environ. Sci. Technol. Lett., vol. 4, no. 3, pp. 105–111, 2017.
[13] H. Zhang, R. Vestergren, T. Wang, J. Yu, G. Jiang, and D. Herzke, “Geographical Differences in Dietary Exposure to Perfluoroalkyl Acids between Manufacturing and Application Regions in China,” Environ. Sci. Technol., vol. 51, no. 10, pp. 5747–5755, 2017.
[14] X. C. Hu et al., “Detection of poly-and perfluoroalkyl substances (PFASs) in US drinking water linked to industrial sites, military fire training areas, and wastewater treatment plants,” Environ. Sci. Technol. Lett., vol. 3, no. 10, pp. 344–350, 2016.
[15] F. A. Ubel, S. D. Sorenson, and D. E. Roach, “Health status of plant workers exposed to fluorochemicals - a preliminary report,” Am. Ind. Hyg. Assoc. J., vol. 41, no. 8, pp. 584–589, Aug. 1980.
[16] P. Grandjean et al., “Estimated exposures to perfluorinated compounds in infancy predict attenuated vaccine antibody concentrations at age 5-years,” J. Immunotoxicol., vol. 14, no. 1, pp. 188–195, Jan. 2017.
[17] M. N. Mead, “Contaminants in Human Milk: Weighing the Risks against the Benefits of Breastfeeding,” Environ. Health Perspect., vol. 116, no. 10, Oct. 2008.
[18] J. M. Armitage, M. MacLeod, and I. T. Cousins, “Modeling the Global Fate and Transport of Perfluorooctanoic Acid (PFOA) and Perfluorooctanoate (PFO) Emitted from Direct Sources Using a Multispecies Mass Balance Model,” Environ. Sci. Technol., vol. 43, no. 4, pp. 1134–1140, Feb. 2009.
[19] M. F. Rahman, S. Peldszus, and W. B. Anderson, “Behaviour and fate of perfluoroalkyl and polyfluoroalkyl substances (PFASs) in drinking water treatment: A review,” Water Res., vol. 50, pp. 318–340, Mar. 2014.
[20] F. Wong et al., “Assessing temporal trends and source regions of per- and polyfluoroalkyl substances (PFASs) in air under the Arctic Monitoring and Assessment Programme (AMAP),” Atmos. Environ., vol. 172, pp. 65–73, Jan. 2018.
[21] E. Hepburn, C. Madden, D. Szabo, T. L. Coggan, B. Clarke, and M. Currell, “Contamination of groundwater with per- and polyfluoroalkyl substances (PFAS) from legacy landfills in an urban re-development precinct,” Environ. Pollut., vol. 248, pp. 101–113, May 2019.
[22] E. Kissa, Fluorinated surfactants and repellents, vol. 97. CRC Press, 2001.
[23] R. Renner, “The long and the short of perfluorinated replacements,” Environ. Sci. Technol., vol. 40, no. 1, pp. 12–13, Jan. 2006.
[24] S. C. Gordon, “Toxicological evaluation of ammonium 4,8-dioxa-3H-perfluorononanoate, a new emulsifier to replace ammonium perfluorooctanoate in fluoropolymer manufacturing,” Regul. Toxicol. Pharmacol., vol. 59, no. 1, pp. 64–80, 2011.
[25] Z. Wang, I. T. Cousins, M. Scheringer, and K. Hungerbuehler, “Hazard assessment of fluorinated alternatives to long-chain perfluoroalkyl acids (PFAAs) and their precursors: Status quo, ongoing challenges and possible solutions,” Environ. Int., vol. 75, pp. 172–179, Feb. 2015.
[26] J. Lijzen, “Mixture exposure to PFAS: A Relative Potency Factor approach,” National Institute for Public Health and the Environment, RIVM Report 2018-0070.
[27] USEPA, “Perfluoroalkyl Sulfonates; Significant New Use Rule,” Fed. Regist., vol. 67, no. 236, pp. 72854–72867, 2002.
Additional efforts in California by other CalEPA agencies are included below:
PFCAs | PFSAs | FOSAA | FOSA | FTS | FTCA | FASE | FASA | PFECA
Perfluoroalkylcarboxylic acids (PFCAs)
| Chemical Name | Abbreviation | Chemical Abstracts Service (CAS) No. |
|---|---|---|
| Perfluorohexanoic acid | PFHxA | 307-24-4 |
| Perfluorododecanoic acid | PFDoDA | 307-55-1 |
| Perfluorooctanoic acid | PFOA | 335-67-1 |
| Perfluorodecanoic acid | PFDA | 335-76-2 |
| Perfluorobutanoic acid | PFBA | 375-22-4 |
| Perfluoroheptanoic acid | PFHpA | 375-85-9 |
| Perfluorononanoic acid | PFNA | 375-95-1 |
| Perfluorotetradecanoic acid | PFTeDA | 376-06-7 |
| Perfluoroundecanoic acid | PFUnDA | 2058-94-8 |
| Perfluoropentanoic acid | PFPeA | 2706-90-3 |
| Perfluorotridecanoic acid | PFTrDA | 72629-94-8 |
| Perfluorohexadecanoic acid | PFHxDA | 67905-19-5 |
| Perfluorooctadecanoic acid | PFODA | 16517-11-6 |
Perfluorinated sulfonic acids (PFSAs)
| Chemical Name | Abbreviation | Chemical Abstracts Service (CAS) No. |
|---|---|---|
| Perfluorodecane sulfonic acid | PFDS | 335-77-3 |
| Perfluorohexane sulfonic acid | PFHxS | 355-46-4 |
| Perfluorobutane sulfonic acid | PFBS | 375-73-5 |
| Perfluoroheptane sulfonic acid | PFHpS | 375-92-8 |
| Perfluorooctane sulfonic acid | PFOS | 1763-23-1 |
| Perfluoropentane sulfonic acid | PFPeS | 2706-91-4 |
| Perfluorononane sulfonic acid | PFNS | 474511-07-4 |
| 9-Chlorohexadecafluoro-3-oxanonane-1-sulfonic acid | 9-Cl-PF3ONS | 756426-58-1 |
| 11-Chloroeicosafluoro-3-oxaundecane-1-sulfonic acid | 11-Cl-PF3OUdS | 763051-92-9 |
Perfluorinated sulfonamidoacetic acids (FOSAA)
| Chemical Name | Abbreviation | Chemical Abstracts Service (CAS) No. |
|---|---|---|
| N-Methyl perfluorooctane sulfonamidoacetic acid | NMeFOSAA | 2355-31-9 |
| N-Ethyl perfluorooctane sulfonamidoacetic acid | NEtFOSAA | 2991-50-6 |
Perfluorinated sulfonamides (FOSA)
| Chemical Name | Abbreviation | Chemical Abstracts Service (CAS) No. |
|---|---|---|
| Perfluorooctanesulfonamide | PFOSAm | 754-91-6 |
Fluorotelomer sulfonates (FTS)
| Chemical Name | Abbreviation | Chemical Abstracts Service (CAS) No. |
|---|---|---|
| 6:2 Fluorotelomer sulfonic acid | 6:2 FTS | 27619-97-2 |
| 8:2 Fluorotelomer sulfonic acid | 8:2 FTS | 39108-34-4 |
| 4:2 Fluorotelomer sulfonic acid | 4:2 FTS | 757124-72-4 |
| 10:2 Fluorotelomer sulfonic acid | 10:2 FTS | 120226-60-0 |
Fluorotelomer carboxylic acids (FTCA)
| Chemical Name | Abbreviation | Chemical Abstracts Service (CAS) No. |
|---|---|---|
| 2H,2H,3H,3H-Perfluorohexanoic acid | 3:3 FTCA | 356-02-5 |
| 2H,2H,3H,3H-Perfluorooctanoic acid | 5:3 FTCA | 914637-49-3 |
| 2H,2H,3H,3H-Perfluorodecanoic acid | 7:3 FTCA | 812-70-4 |
Perfluoroalkane sulfonamido ethanols (FASE)
| Chemical Name | Abbreviation | Chemical Abstracts Service (CAS) No. |
|---|---|---|
| N-Ethyl perfluorooctane sulfonamide ethanol | EtFOSE | 1691-99-2 |
| N-Methyl perfluorooctane sulfonamide ethanol | MeFOSE | 24448-09-7 |
Perfluoroalkane sulfonamides (FASA)
| Chemical Name | Abbreviation | Chemical Abstracts Service (CAS) No. |
|---|---|---|
| N-Ethyl perfluorooctane sulfonamide | EtFOSA | 4151-50-2 |
| N-Methyl perfluorooctane sulfonamide | MeFOSA | 31506-32-8 |
Perfluoroether carboxylic acids (PFECA)
| Chemical Name | Abbreviation | Chemical Abstracts Service (CAS) No. |
|---|---|---|
| Hexafluoropropylene oxide dimer acid | HFPO-DA | 13252-13-6 |
| 4,8-Dioxa-3H-perfluorononanoic acid | ADONA | 919005-14-4 |
Subscribe to our Per- and Polyfluoroalkyl Substances (PFAS) email list to receive notifications and the latest updates. After subscribing, you will need to check your email host for a confirmation email to complete the subscription.
Subscribe to our other email lists. See the "Water Quality" section.
Quick Links
Questions? Comments?
If you have questions about our program, please email us at:
For additional information about PFAS
- Division of Drinking Water District Engineers
- GeoTracker Help Desk
- ITRC PFAS Fact Sheets (English and Spanish)
- Regional Water Board Contacts
- US EPA PFAS Website
- San Diego Regional Water Quality Control Board — PFAS website
- San Francisco Regional Water Quality Control Board — PFAS website
- Santa Ana Regional Water Quality Control Board — PFAS website
- Los Angeles Regional Water Quality Control Board – PFAS website
Other PFAS Resources
- US Navy Administrative Record
- US Air Force Administrative Record
- SERDP/ESTCP – DoD’s Environmental Research Programs
- Feb 14, 2019: US EPA Announces PFAS Action Plan
- DoD Quality Systems Manuals
- Agency for Toxic Substances and Disease Registry (ATSDR)
- United States Environmental Protection Agency (US EPA)
- Interstate Technology & Regulatory Council (ITRC)


